Currents
I Promise We Don’t Want Your Business… Yet
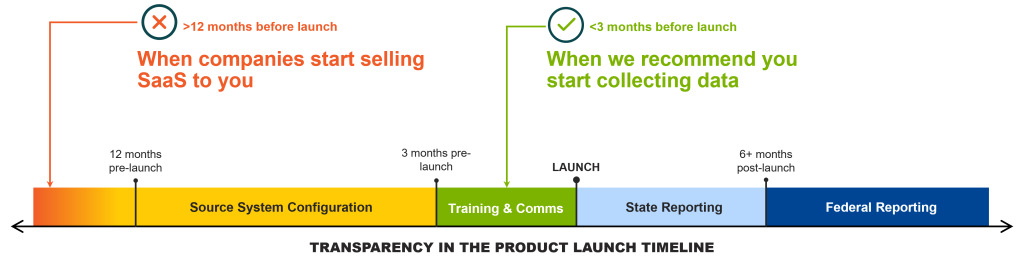
Over the last year, I’ve been on at least five calls with companies where I’ve said, “I don’t think you need Potomac’s support, save your money for now.” Each time, the companies have been more than a year away from launching their products and are already asking about deploying a software solution to meet their Sunshine Act reporting obligations.
Don’t get over sold… if you’re more than a year away from launch, you do not need to purchase a software solution for transparency reporting. That’s far too early. Don’t rush.
When do I need to begin collecting data? When do I file my first report?
FDA approval is a milestone worth celebrating, so enjoy it! But it also exposes your company to a whole new set of compliance obligations. If your product is reimbursed by Medicare, Medicaid, or CHIP, you have a Covered Product and are very likely on your way to being considered an Applicable Manufacturer under the Sunshine Act.
There’s some good news: designation as a new Applicable Manufacturer qualifies for a 180-day grace period from when the product becomes first available for purchase before manufacturers are obligated to start CMS data capture. Therefore, the earliest payment that you’ll report to CMS is at least six months after launch.
Keep in mind that many of the states aren’t quite so accommodating and do not have the same 180-day grace period. So, depending on the industry (rules vary between pharma and device) and states in which you operate, you may have state reporting obligations at launch.
A Roadmap for Implementation
Capturing and reporting data for Sunshine reporting may seem like a daunting task but it doesn’t have to be a full-time job and you don’t need complicated or expensive systems to fulfil your reporting obligations. I’ve helped numerous companies launch their transparency programs and each company faces a different set of challenges when launching. Following these steps can help you better prepare:
Step One: Examine Your Spend
First, critically examine your internal infrastructure and determine if your existing framework has the capacity to capture appropriate data. Conducting a comprehensive spend inventory will help you prepare for data capture and the launch of a transparency system. A spend inventory offers clear insights into your spending and about the process you plan on using to capture the data. This doesn’t mean you always need to integrate an IT system; in many cases, Excel works just fine.
Step Two: Configure Source Systems
Not all life sciences companies are the same and neither are their source systems! For new companies, the first system I recommend focusing on is your highest-volume system, usually the T&E system (most frequently Concur in the life sciences industry). Implementing a tool to allow users to search for HCPs within your T&E system instead of manually entering information is one of the best investments you can make in the early stages. It will save you and your team headaches and time during reporting. Beyond T&E, it’s helpful to have a plan on how you will capture other areas of spend such as ERP direct payments, third-party vendor payments, reprints, and any other area identified in Step One.
Step Three: Determine Your Best Reporting Option
For most companies, investing in a flashy new system or hiring full-time support can be time-consuming and costly when resources are limited and there aren’t enough hours in the day to complete everything needed for launch. In my experience, most companies simply need to analyze their existing infrastructure and procure supplemental resources or external assistance to fill the gaps.
How Should my Approach to Transparency Change Based on Organization Size?
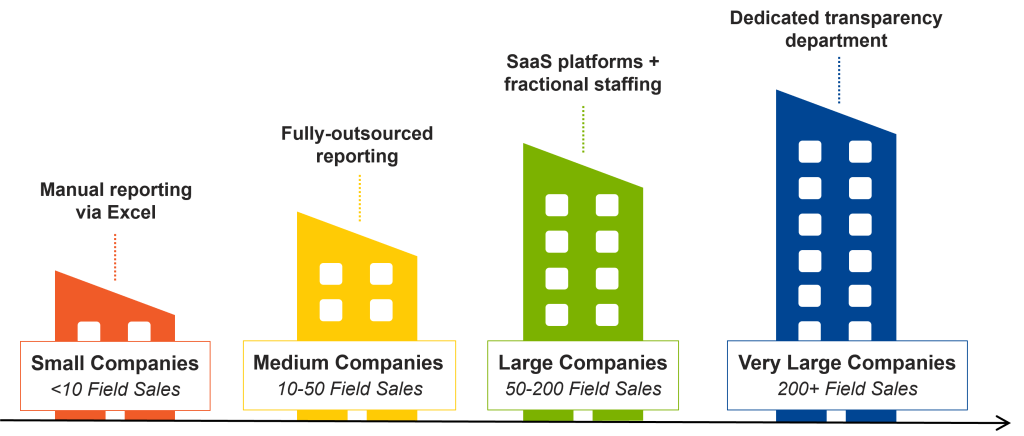
Level 1 (<10 Reps): Smart Manual Solutions
For companies with a sales force of 10 representatives or fewer and minimal reportable transactions, your entire CMS reporting can likely be handled via Excel. There are also companies that focus on manual reporting options at extremely cost-effective prices.
Level 2 (10-50): Managed Services
Companies with 10-50 representatives often have enough complexity to need real infrastructure but not enough to justify a large technology investment in a SaaS solution. Hiring a managed services firm for outsourced support is an efficient and simple approach to fulfilling reporting obligations. Managed service firms help companies with limited volume navigate data management. For example, my team at Potomac uses our in-house aggregate spend database, WholeSum, to collect, remediate, and report your data without the overhead and effort associated with implementing and running SaaS Transparency solutions.
Level 3 (50-200): Fractional Support
For higher volume companies, reporting can be streamlined by using SaaS solutions that automates data collection from various sources, generates reports, and provides the company with greater access to their data for monitoring and other purposes. However, a SaaS system does not necessarily require a full-time transparency resource. There are companies, including Potomac, that provide fractional transparency team support to allow your team to focus their attention on other areas of your compliance program. Transparency experts are typically the busiest during “reporting season,” the period from January-March before Open Payment reports are due. But there is often less to do throughout the year, making it difficult to justify a full-time headcount.
Level 4 (200+): Transparency FTE
If your company has over 200 representatives, you will likely need to build out a larger-scale transparency program that likely includes licensing a SaaS solution and hiring a full-time resource. There are still decisions to be made about the seniority level of the employee and whether they are primarily leading the program with support from others or with a data steward who is an expert at getting data into the SaaS system, remediating errors, and analyzing data for accuracy.
The Grace Period (And How to Use it Wisely)
Required reporting begins after the 180-day grace period between March and July 1 of the year following approval. With no need for retrospective reporting under the Sunshine Act, the grace period gives you the time to build a strong Transparency Reporting foundation.
This is a time to practice collecting data, fix processes, and refine your approach. Use this time wisely and treat it as a test run. Use real data and confirm if what you collected was complete.
Was the process working? Are there areas for improvement?
Use this grace period to find issues now instead of right before reporting deadlines.

Don’t Overcomplicate It
Getting FDA approval for your first product is exciting! I know that comes with pressure and that pressure is real. But launching a transparency program doesn’t have to be a lonely (or expensive) sprint. The best programs are the ones that start simply, build steadily, and fit the solution to their company. Don’t get oversold…