Currents
A Smarter Way to Build Your Monitoring Plan
When life sciences compliance teams build monitoring plans, they often start with a broad list of activities such as speaker programs, advisory boards, and charitable contributions.
But here’s the problem: these activities are often conducted in different ways, resulting in various layers of risks depending on the way it’s being done. Consider speaker programs as an “umbrella activity” with those layers underneath. Under the umbrella might include live programs, unbranded disease state programs, programs related to one therapeutic area versus another… the list goes on. When a compliance officer begins to consider the variations of each umbrella activity, it might lead to the question of how to deploy monitoring resources to address the highest risk activities.
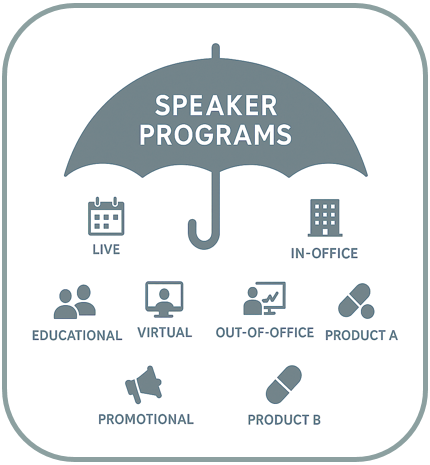
Start with the Activity – Then Go Deeper
At first glance, it might seem like your compliance team should start with regulatory risks like kickback or off-label promotion when developing a monitoring plan. But Potomac has found it’s more effective to begin by understanding the full scope of what your teams are doing. Listing out activities serves as the foundation for identifying risk and can complement your compliance risk assignment to directly align to your monitoring plan.
Once you have your list of “umbrella activities”, ask yourself and your team: Are the risks different, even within the same type of activity?
The answer is almost always yes. And that’s where segmentation comes in.
Why Segmentation Matters
Segmentation means breaking down your umbrella activities into smaller categories that reflect different risk profiles. That could include:
- Format (live vs. virtual)
- Audience (patients or HCPs)
- Product profile (new launch vs. established; high off-label use or not)
- Varying risk tolerance across business units
- Geography (U.S. & international, regions/territories, etc.)
For example, live speaker programs for a newly launched therapeutic area might require closer scrutiny than a mature brand presenting data on a new indication.
By segmenting, you’re no longer saying, “We’ll monitor 10% of speaker programs.” You’re defining which ones, why, and how often based on a defensible risk rationale. Segmentation becomes increasingly important for mid-size and large life sciences companies considering the high volume of business activities. As an added benefit, this approach helps ensure your monitoring team work efficiently while focusing on the most critical compliance risks
Visualize the Approach
The graphic below illustrates how a broad activity like “speaker programs” can be broken down into manageable, meaningful segments. Applying multiple layers of segmentation allows you to hone in on the most relevant risks and prioritize with greater precision.
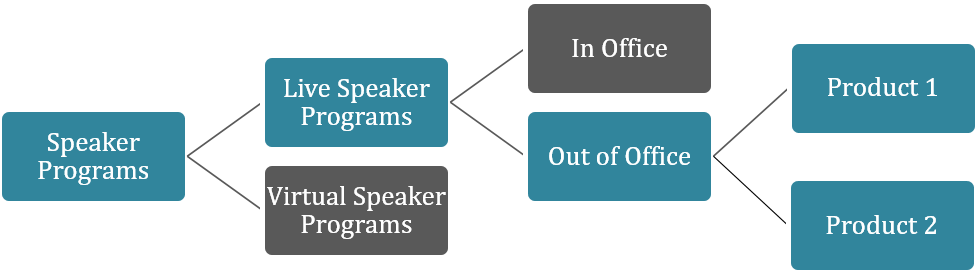
Even if you don’t have exact volumes while segmenting your activities, that’s okay. Use historical data, your annual needs assessment, annual budget plans, and business strategy to make informed estimates and update your plan as you learn more about activity volumes.
Build a Flexible Plan
Once you’ve identified your high-risk segments, build a plan that balances:
- Risk-based reviews (targeted where you’re most vulnerable)
- Randomized sampling (to catch blind spots and establish baselines)
Then make segmentation and risk review an annual exercise. Risks shift. Controls evolve. Revisiting your segmentation regularly keeps your monitoring plan relevant and proactive.
Use Monitoring as a Strategic Tool
With an organized, segmented approach, your monitoring plan becomes more than just checking the box on compliance requirements. Your compliance program is becoming more effective, turning monitoring into a tool that helps you understand and gain visibility into your business activities, detect and mitigate risks, and strengthen your controls over time.
So, the next time you revisit your compliance program plan and monitoring goals, don’t just ask what you need to review – ask how you can segment smarter to focus on what matters most!
Potomac is Here to Help Bring Clarity
“Umbrella activities”, segmentation, selections – oh my! Potomac is excited to cover the key concepts of segmented monitoring plans at PCF 2025.
Join me alongside a panel of pharmaceutical and medical device industry experts to learn more about building a smarter monitoring plan.